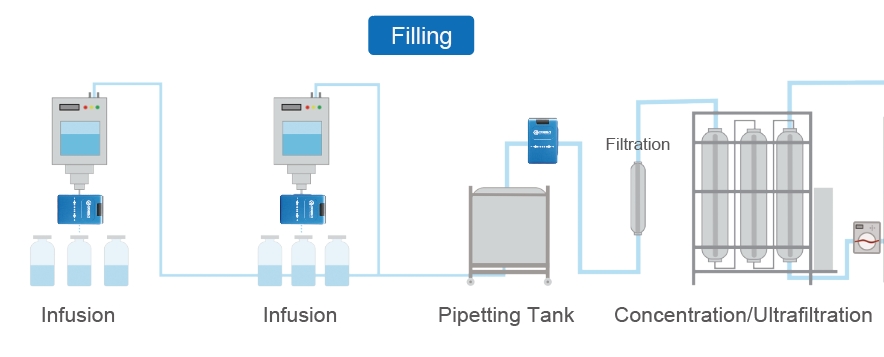
Aseptic filling is a critical process in the pharmaceutical and biotechnology industries, ensuring that sterile products such as vaccines, injectables, and biologics are safely and effectively packaged without contamination.
This process involves maintaining a sterile environment throughout the entire operation—from the preparation of the fill solution to the final packaging of the product.
Aseptic filling is vital for preserving the integrity, efficacy, and safety of pharmaceutical products, making adherence to stringent industry standards and regulations crucial.































 Pre-Sale
Pre-Sale After-Sale
After-Sale